Are you looking for someone to “write my lab report“? We have tutors in most of the subjects if not all. Click the button below to post your lab report details and we will advise further.
Discussion Questions
- What was the purpose of this experiment?
- What is the hypothetical equation for the reaction of Fe2+ and ortho-phenanthroline?
- When preparing the samples, the amount of stock B (Fe+2) was constant but the amount of
ortho-phenanthroline increased.
- Looking at the picture below, what do you notice about the colors of the samples as you go from #1 to #8 ?
- How does this observation compare with what was expected?
- How was this observation evident on the graph?
- How can you explain what happens to the absorbance of samples #2 – #5?
- How can you explain what happens to the absorbance for the last 3 samples?
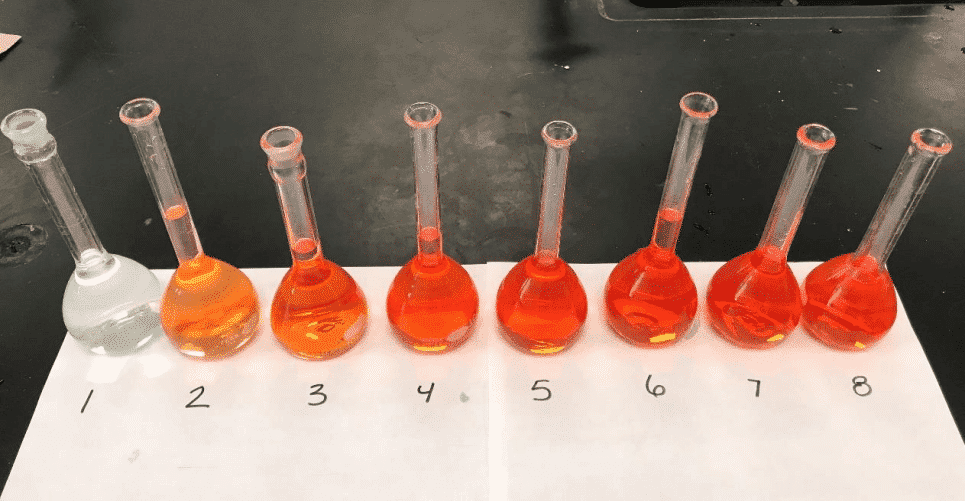
ortho-phenanthroline increased with the amount of stock B (Fe+2) remaining constant
- The “n” value is the mole ratio for the balanced equation. Mole ratios in a balanced equation
are whole number values (i.e integers). Write the full balanced equation for the reaction
between Fe+2 and ortho-phenanthroline using in your result for “n”.
- The true result for n = 3. Calculate the % error. Compare your experimental result to this value and comment on the comparison.
% error = [(𝑎𝑐𝑡𝑢𝑎𝑙 𝑣𝑎𝑙𝑢𝑒−𝑒𝑥𝑝𝑒𝑟𝑖𝑚𝑒𝑛𝑡𝑎𝑙 𝑣𝑎𝑙𝑢𝑒)/ 𝑎𝑐𝑡𝑢𝑎𝑙 𝑣𝑎𝑙𝑢𝑒] x 100
- Discuss 3 possible sources of experimental error and how the errors would affect the results.
Do you have a similar lab report that you help with. Why don’t you consider to “pay someone to write my lab report“. We have the best chemistry tutors waiting to help you. Click the button below and post your assignment instructions.
Final Calculations
You should complete and submit all of the following calculations. Remember, a complete calculations section includes:
- Identify what you are calculating
- The equation used for the calculation
- The equation filled in with the data (including units)
- The final result/answer for the calculation (with proper units and sig figs)
For this experiment, you can show your worked out calculations in the space provided or on a separate page.
This first set of calculations deals with the Fe+2 solutions used in the experiment.
Stock A Molarity: (Note: 0.XXXX grams comes from the data pic for grams of ferrous Av
MA =
Stock B Molarity: (note: VA = 25.00 mL (volume of the pipet) and VB = 250.0 mL (volume of the volumetric flask)
MB =
Millimoles of Fe+2 in solutions # 1 – 8: For each solution 10.00 mL of the Stock B solution was pipetted into each flask.
Millimoles of Fe+2 = (10.00 mL) x (MB)
This set of calculations deals with the information from the graph.
Average Absorbance for the Horizontal Data: Determine the average value for the absorbances of the 3 horizontal data points
Average Horizontal Absorbance =
Intersection point of the graph. You should notice on the graph that if a horizontal line is drawn through the horizontal points, it will intersect the increasing trendline. You can calculate the millimoles of ortho-phenanthroline that corresponds to that intersection point by using the y = m x + b trendline equation from the graph.
Where:
- y = your average horizontal absorbance
- m = slope from the trendline
- b = y intercept from the trend line
You can solve for the “x” value – this is the millimoles of ortho-phenanthroline at the intersection
y = m x + b“n” value – this is the ratio of the millimoles of ortho-phenanthroline at the intersection point to the millimoles of Fe+2 in the solutions.
“n’” = Millimoles of ortho-phenanthroline at the the intersection point / millimoles of Fe+2 in each solution
Our lab report writers are just a click away. Click the button below and let us help you improve your class performance.
General Graph Format
- The graph should be a full page (8 ½ x 11)
- The plotted points should take up most of the page (adjust x or y axis scaling if necessary).
- Clearly show all data points
- Make sure to label both axes with the variable and units
- Title the graph correctly. The title for the graph is NOT the title of the experiment. The title of the graph is “y-variable” vs. “x-variable”… Fill in the proper labels for the x & y values.
- Don’t connect the dots when a slope and intercept are determined from a best fit line. Use excel to put in a trendline.
HOW TO DRAW THE GRAPH IN EXCEL (2016) For The Stoichiometry Of A Metal Complex Experiment
Note: This graph will have 2 sets of data plotted and one best fit line with the y = m x + b equation
- Type in the data/calcs from your worksheet table for solutions # 2 – 8; You will NOT plot solution #1 and do not include units or labels, just the numbers.
- first column = X axis (millimoles of ortho-phenanthroline)
- second column = Y axis (absorbance)
- Highlight only the x & y data for the increasing data (solutions # 2 – 5) & click on the INSERT tab along the top
- In the CHARTS box, choose SCATTER (with only markers – top left) for the chart type. This will automatically show the graph for the 4 data points that were highlighted.
- Double click on the [chart title] and re-type the actual title for this graph. (Remember the title is the (y vs x) actual varible labels for this graph)
- Click inside the graphed area and then click on the [+] at the upper right corner of the graph (this allows you to add chart elements). Be sure the following 5 items are checked:
- Axes
- Axis titles
- Chart title
- Gridlines
- Legend
- For AXIS TITLES – this puts generic axis titles for both the x & y axes
- Double click on each [Axis title] and re-type the actual axis label into each.
- Next, you will add in a second set of data.
- Right-click inside the middle of your graph and choose [Select Data] then [Add]
- Fill in the [Series Name] as “Horizontal”
- For the [Series X values], click on the red arrow to the right, highlight the millimoles for solutions # 6 – 8, click on the red arrow to get back to [Edit Series]
- Now for the [Series Y values] click on the red arrow to the right, and highlight the 3 Absorbance values for solutions # 6 – 8, then click [OK] to finish the [Edit Series]
- You should see 2 series of data listed – Series 1 should be renamed. Click on Series 1 to highlight it, and then click on [Edit]; you can then type “Increasing” into the name box; then click [OK] to close out the Edit box and then [OK] again to close out the select data source box
- This should show a second set of data points in a different color/shape than the first full set of data and a legend that identifies the increasing and horizontal data
- Now to add in the best-fit trendline for that first set of data (the increasing points). Right-Click on any one of the 1st set of increasing data points and all of those 4 points should be highlighted. Now choose ADD TRENDLINE.
- The FORMAT TRENDLINE BOX should open up on the right. Choose and fill in the following:
- Linear
- Forward 0.010 periods
- Backward 0.005 periods
- Display equation
- Click on the [X] at top right when you are done. This will close out the Format Trendline Box
- This will show and extend the best-fit line and the equation for y = mx + b should appear on the graph. You can move the equation if it overlaps data or a gridline..
- Finally, to make the graph a full page, you can [move chart]. On the far top right, click on MOVE CHART (location) and choose New Sheet and click [OK]. Or you can right click in the white area to the right of the graph and get to [move chart] and choose “New Sheet”.
- Save the graph as a pdf so that it can be uploaded to BB.
No need to wait till last minute to make the right decision. We have a chemistry homework solver just a click away. Click the button below to get chemistry lab report help.
Would you want to speak to one of our support agents to help you place your order? We are on WhatsApp always. We are also online on chat.